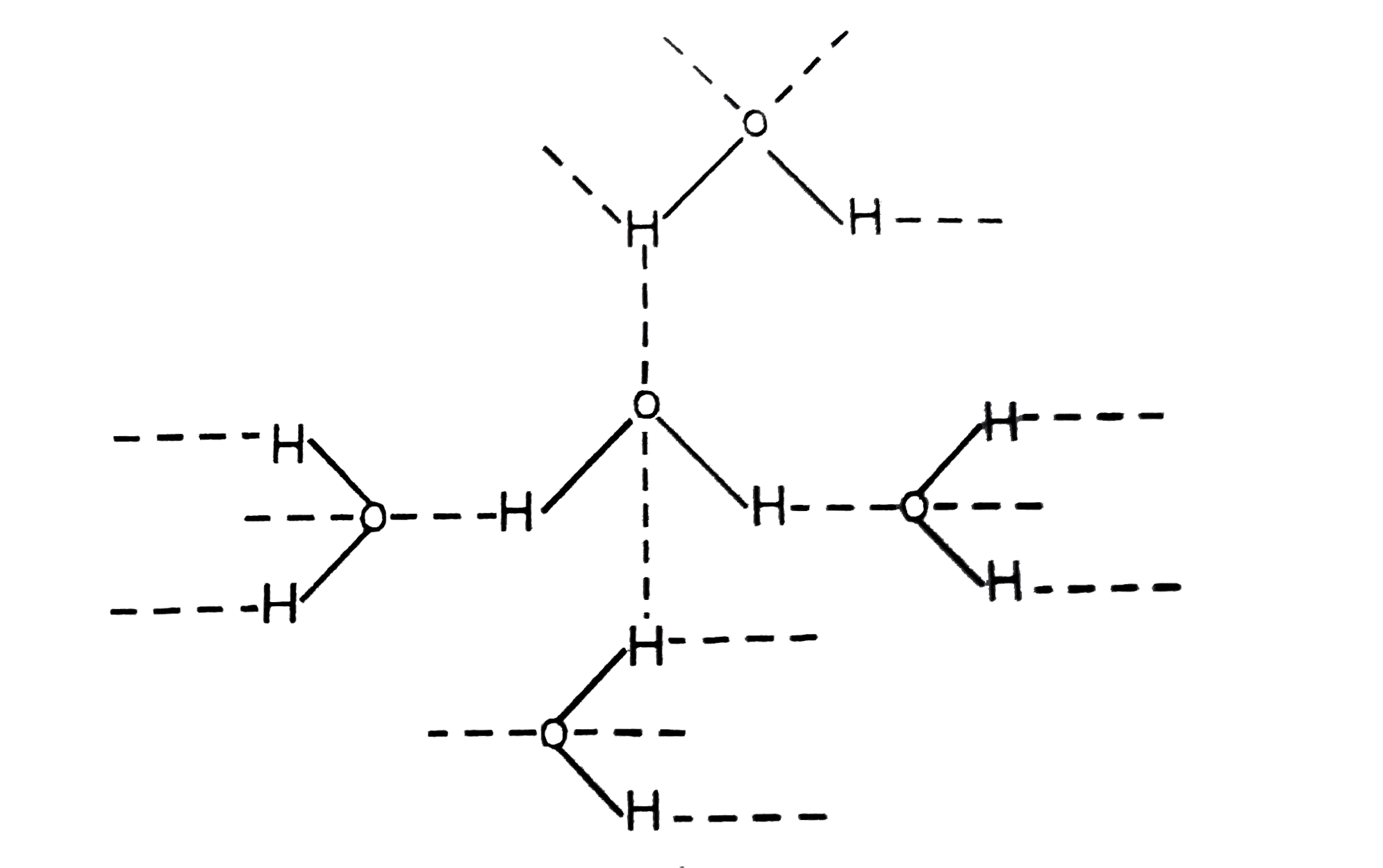
The water molecule has a stronger dipole a negatively and positively charged end to the individual molecule so these negative and positive ends continually attract each other like magnets of opposite poles creating more cohesion between the molecules of water explaining the liquid phase.
Why water is a liquid at room temperature but hydrogen sulphide is a gas.
H2s hydrogen sulfide is a gas because at room temperature the forces and interactions between the molecules of hydrogen sulfide are very weak.
Thus due to stronger intermolecular forces in water water will be a liquid and hydrogen sulfide having much weaker intermolecular forces will therefore exist as a gas under standard atmospheric.
Answered by melissa k.
Although hydrogen sulphide has a greater molecular mass than water water is a liquid at room temperature while carbon dioxide is a gas because of the strong intermolecular forces that hold water.
Paper by super 30 aakash institute powered by embibe analysis improve your score by 22 minimum while there is still time.
Have you registered for the pre jee main pre aipmt 2016.
This energy is available at room temperature and hence hydrogen sulphide is a gas while water is still a liquid.
The water molecule has a stronger dipole a.
Its a very strong bond that packs the water molecules closely together forming a liquid.
Hence water has a higher boiling point or is a liquid at room temperature whereas h 2 s is a gas at room temperature.
So lesser energy is required to overcome the forces of interaction between the hydrogen sulphide molecules than those between water molecules.